Boosters for Moderna and Johnson & Johnson
The Centers for Disease Control and Prevention (CDC) last week officially endorsed booster shots for Moderna and Johnson & Johnson COVID-19 vaccines for certain populations. Pfizer boosters were already endorsed last month.
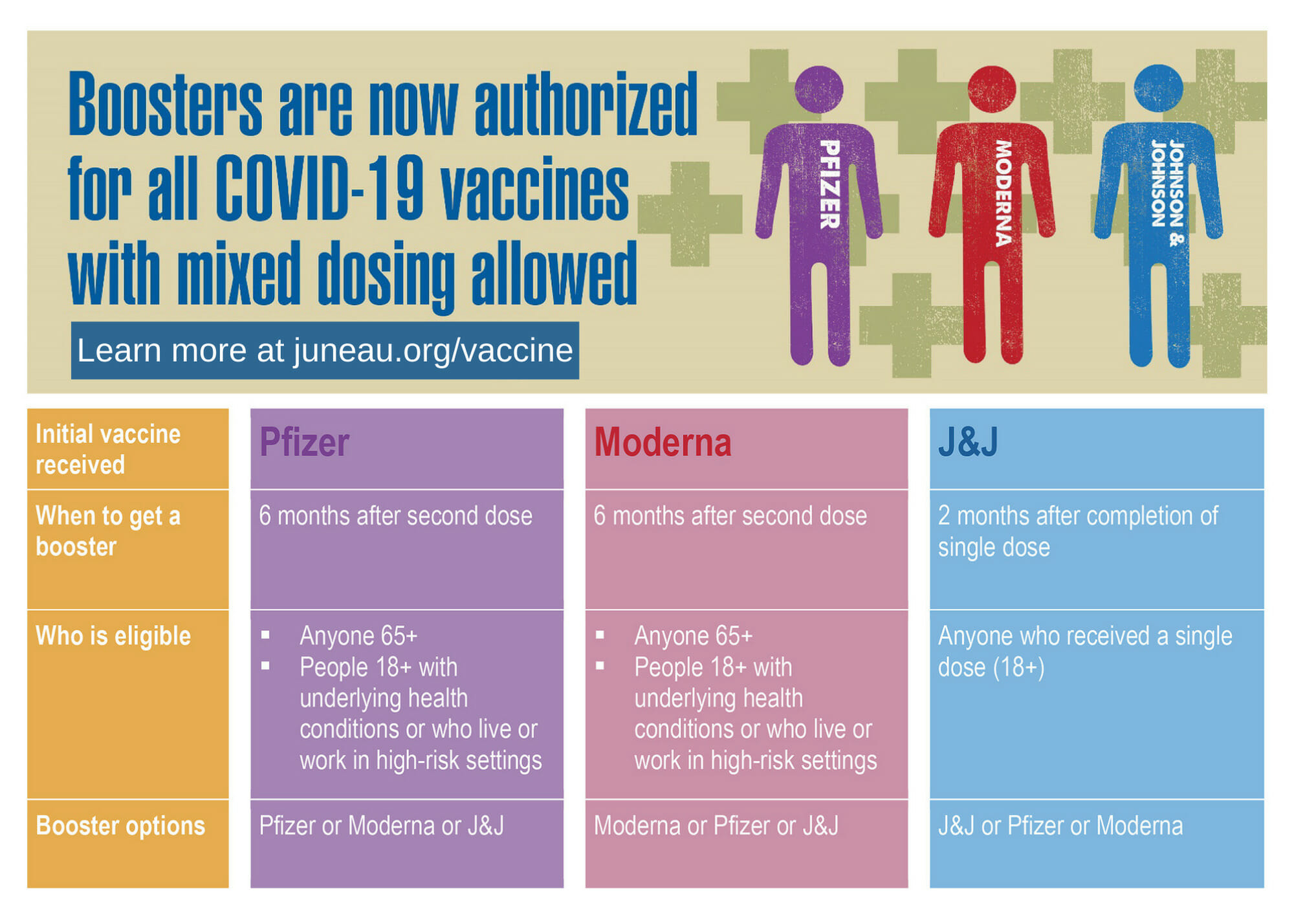
For individuals who received a Pfizer or Moderna COVID-19 vaccine, the following groups are eligible for a booster shot 6 months or more after their initial series:
- 65 years and older
- Age 18+ who live in long-term care settings
- Age 18+ who have underlying medical conditions
- Age 18+ who work in high-risk settings, like first responders (e.g., healthcare workers, firefighters, police, congregate care staff); education staff (e.g., teachers, support staff, daycare workers); food and agriculture workers; manufacturing workers; corrections workers; U.S. Postal Service workers; public transit workers; grocery store workers
- Age 18+ who live in high-risk settings
For individuals who received the Johnson & Johnson COVID-19 vaccine, booster shots are recommended for those who are 18 and older, and who were vaccinated two or more months ago. There are no other eligibility requirements.
Eligible individuals may choose which vaccine they receive as a booster dose. Some people may have a preference for the vaccine type they originally received while others may prefer to get a different booster. CDC’s recommendations now allow for this type of mix and match dosing for booster shots. That means:
- Eligible individuals who received a Pfizer or Moderna COVID-19 vaccine can get a booster dose of Pfizer, Moderna, or Johnson & Johnson 6 months after their initial vaccine series.
- Individuals ages 18 and up who received a Johnson & Johnson COVID-19 vaccine can get a booster dose of Pfizer, Moderna, or Johnson & Johnson 2 months after their initial dose.
For more information, email [email protected].
